2021-09-17
In September, the final meeting of the project "Randomized, controlled, and multicenter clinical study on the efficacy and safety of Shengbai oral liquid for the prevention/treatment of neutropenia after chemotherapy in patients with non-small cell lung cancer" was held in Beijing on September 10, 2021.
Professor Lin Hongsheng, PI of the project and chief researcher of Guang'anmen Hospital of China Academy of Chinese Medical Sciences, Liu Jie, director of Guang'anmen Hospital of China Academy of Chinese Medical Sciences, and Professor Shi Qiuling of Chongqing Medical University attended and participated in the meeting. Mr. Zhang Min, Chairman of Hubei Mengyang Pharmaceutical Co., Ltd., the sponsor of the project, Ms. Zhou Huiying, Deputy General Manager, and Ms. Huang Qian, Vice President of R&D, all attended the meeting. The meeting was presided over by Ms. Meng Rui, project PM, Beijing Yijia Medical Pharmaceutical Technology Co., Ltd.
As a drug for the treatment of leukopenia caused by radiotherapy and chemotherapy for cancer, Shengbai Oral Liquid is safe and effective in clinical application, and the efficacy can be evaluated by quantitative indicators (white blood cell count), and has been included in the "13th Five-Year Plan" textbook of higher education in the national traditional Chinese medicine industry, "Hematology of Traditional Chinese Medicine" and "Interpretation of Traditional Chinese Medicine Diagnosis and Treatment Plans and Pathways for Dominant Diseases of Blood Diseases", "Interpretation of Clinical Pathway Therapeutic Drugs, Tumor Diseases", and "Interpretation of Clinical Pathway Therapeutic Drugs: Hematological Diseases"; It is included in the "Guidelines for Traditional Chinese Medicine Diagnosis and Treatment of Malignant Tumors" and "Expert Consensus on Palliative Treatment of Tumors".
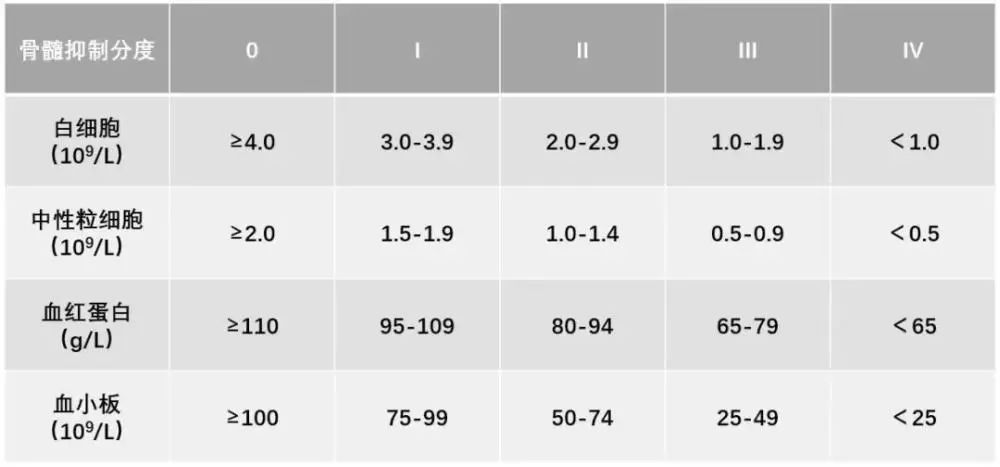
In order to further evaluate the effectiveness and safety of Shengbai Oral Liquid in the prevention/treatment of neutropenia after chemotherapy in patients with non-small cell lung cancer, provide a basis for decision-making on the rational allocation of medical resources, and provide guidance for the rational use of drugs in clinical practice, in 2018, Mengyang Pharmaceutical Co., Ltd., together with 21 grade hospitals, conducted a multi-center clinical study on the post-marketing re-evaluation of Shengbai Oral Liquid. After three years, 238 patients with non-small cell lung cancer treated with platinum-containing two-drug chemotherapy regimen were screened and enrolled, and were divided into the experimental group and the control group according to the 1:1 enrollment cases, the control group was treated with platinum-containing (carboplatin/cisplatin) two-drug chemotherapy, and the experimental group was treated with raw white oral liquid + platinum-containing (carboplatin/cisplatin) two-drug chemotherapy. There were no significant differences in gender, age, ethnicity, weight, height, allergy history, disease diagnosis, previous treatment history, comorbidities, and ECOG score. While observing the effects of raw white oral liquid on the incidence, occurrence and recovery time of myelosuppression, the effect on cancer-related fatigue was also added, and fingerprinting and ctDNA detection were added to the RCT study for the first time in the post-marketing re-evaluation of proprietary Chinese medicines.
Through multi-party verification, we have seen the effective efficacy of raw white oral liquid in the prevention/treatment of neutrophilpenia after chemotherapy in patients with non-small cell lung cancer, and observed that it also has a good preventive effect on thrombocytopenia, and has shown significant advantages in symptom improvement, especially the improvement of fatigue symptoms. The high-level evidence-based medical evidence also made Shengbai Oral Liquid successfully included in the "Expert Consensus on the Diagnosis and Treatment of Bone Myelosuppression Caused by Antineoplastic Drugs".

COPYRIGHT© 2022 Hubei Mengyang Pharmaceutical Co., LTD. All Rights Reserved E ICP 11014915; Internet Drug Information Service Certificate No. : (E) - Non-business -2016-0032
Technical Support: SUMA