2019-02-18
(发表于《临床肿瘤学杂志》2019年1月第24卷 第1期 P54-59)

Funds: Zhejiang Provincial Key Project of Traditional Chinese Medicine(2015ZZ004)
01
Background:
Chemotherapy is an indispensable way in the treatment of malignant tumors, which plays an important role in prolonging the survival time and improving the quality of life of patients, but it often affects the treatment process because it causes myelosuppression and leukocytosis. Shengbai mixture is an oral preparation made by extracting and concentrating 11 traditional Chinese medicine materials such as epimedium weed, astragalus, angelica, wolfberry, etc., which has the effects of warming the kidneys and strengthening the spleen, nourishing qi and blood, etc., and can promote the recovery of bone marrow hematopoietic function, reduce the toxic effect of chemotherapy drugs, and increase chemotherapy tolerance. There are many studies on the clinical efficacy of Shengbai mixture in the treatment of bone marrow suppression after chemotherapy for malignant tumors, but the research subjects and drug duration of each study are different, and it is uncertain whether this has an impact on the research results. In order to explore the clinical efficacy and safety of myelosuppression after chemotherapy for malignant tumors, this study intends to include a number of clinical studies to systematically analyze the effect of biobai mixture in the treatment of myelosuppression after chemotherapy for malignant tumors, in order to provide reference for clinical use.
02
Objectives:
To systematically evaluate the clinical efficacy of Shengbai mixture in the treatment of bone marrow suppression after chemotherapy, so as to provide a reference for clinical use.
03
Research Methods:
Through a systematic search of the full-text database of China Academic Journals Network, Wanfang Database and the literature published in the Chinese medical biology journals of VIP, the clinical research literature on the efficacy of myelosuppression after chemotherapy in the treatment of malignant tumors was collected, and RevMan 5. Version 0 software was used for meta-analysis.
04
Document inspection search
According to the search strategy, 2483 relevant literatures were initially detected, and a total of 17 articles were included according to the literature inclusion and exclusion criteria after reading the title and abstract and excluding duplicate literature. After reading the full text, a total of 7 articles were included in the literature with clinical case-control as the research object.

05
Findings:
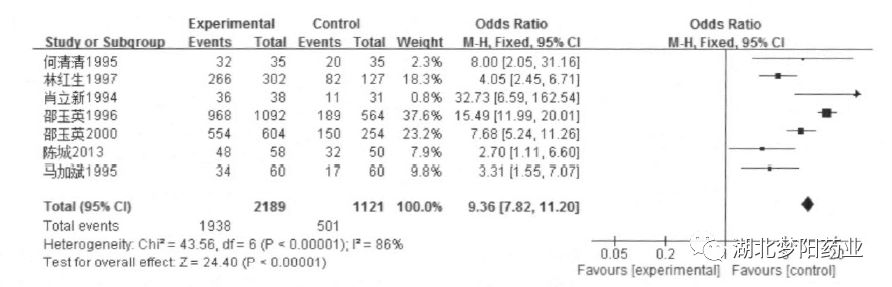
A total of 7 clinical studies with a total of 3310 patients were included. The results of the meta-analysis showed:
Shengbai mixture had obvious clinical efficacy in the treatment of bone marrow suppression after chemotherapy (OR=9.36, 95%CI: 7.82~11.20, P<0.001).
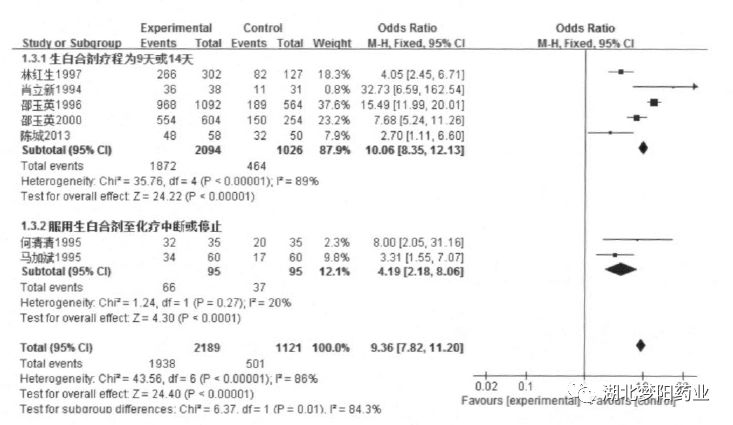
Subgroup analysis showed that the course of treatment with raw white mixture:
9 days (OR=10.06, 95%CI: 8.35~12.13, P<0. 001)
or 14 days (OR=4.19, 95%CI: 2.18~8.06, P<0.001)
The clinical efficacy was better than that of taking raw white mixture until chemotherapy was interrupted or stopped.
Under the two efficacy evaluation criteria, Shengbai mixture can effectively treat bone marrow suppression after tumor chemotherapy
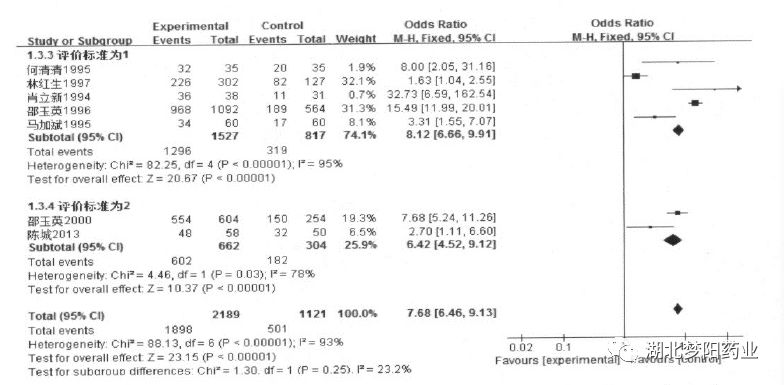
(OR=8.12,95%CI:6.66~9.91,P<0.001;OR=6.42,95%CI :4.52~9.12,P<0.001)
06
Conclusions of the study
Shengbai mixture has obvious clinical efficacy in the treatment of bone marrow suppression after chemotherapy and radiotherapy for malignant tumors, and is safe to use and has no obvious toxic side effects, which is worthy of clinical popularization.





COPYRIGHT© 2022 Hubei Mengyang Pharmaceutical Co., LTD. All Rights Reserved E ICP 11014915; Internet Drug Information Service Certificate No. : (E) - Non-business -2016-0032
Technical Support: SUMA
