2018-04-27
(Published in Chinese Journal of Integrative Medicine and Surgery, April 2018, Vol. 24, No. 2, P131-134)

Foundation Item:
National Natural Science Foundation of China (8140190281501992)
Natural Science Foundation of Hunan Province (2014JJ2144, 2015JJ3086, 2015JJ6064, 2015JJ6065, 2014SK3237, 2015JJ3085)
Most patients with lung cancer are diagnosed at a late stage, with a 5-year survival rate of less than 20%. Chemotherapy is an indispensable part of the comprehensive treatment of lung cancer, and it is also an important treatment method for advanced lung cancer. However, the adverse effects of chemotherapy limit its use, and myelosuppression is the most significant limiting toxicity, especially when the leukocytopenia of grade II and above forces treatment interruption or prolongation. In recent years, Granulocyte colony stimulating factor (G-CSF) has been widely used as the main method for the treatment of severe myelosuppression after chemotherapy. However, the price of G-CSF is high, the injection methods are complicated, and the incidence of side effects such as fever and bone aches is high. This study compared and studied 191 lung cancer patients in Hunan Provincial Cancer Hospital from January 1, 2014 to December 31, 2016, and explored the preventive effect of raw white mixture on bone marrow suppression after chemotherapy.
To observe the protective effect of raw white mixture on bone marrow suppression after chemotherapy for lung cancer.
Inclusion Criteria:
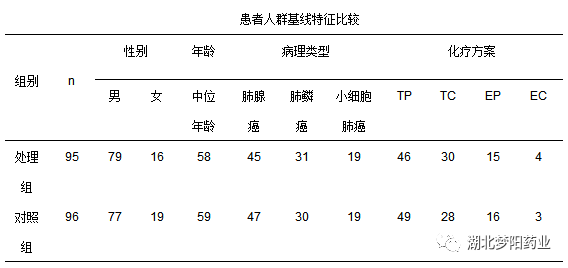
(1) Age≥ 18 years old, ≤ 65 years old, gender is not limited, initial diagnosis and treatment;
(2) Pathologically confirmed non-small cell lung cancer stage IV, small cell lung cancer is extensive, and chemotherapy is treated with TP/TC, EP, EC regimen chemotherapy (chemotherapy drug dose: paclitaxel liposome 175 mg/m2, etoposide 120 mg/m2 or 100 mg/m2, cisplatin 75 mg/m2, D1, carboplatin AUC=6);
(3) The white blood cell ≥ before chemotherapy was 4.0×109/L and ≤ 10×109/L;
(4) PS is 0~1 points, and there is no history of other serious diseases (heart, liver, kidney, blood diseases).
Exclusion Criteria:
(1) Have a history of allergy to this product;
(2) pregnancy;
(3) Use of steroids from the 4th day of chemotherapy to the end of observation;
(4) Concomitant use of other leukocyte-raising drugs other than G-CSF.

Patients with non-small cell lung cancer: paclitaxel liposome 175 mg/m2, D1, cisplatin 75 mg/m2, or carboplatin, AUC=6, every 21 days for a cycle;
Patients with small cell lung cancer: Etoposide 120mg/m2, D1-3, combined with cisplatin 75mg/m2, D1; or etoposide 100 mg/m2, D1-3, plus carboplatin, every 21 days for a cycle.
Shengbai group: start oral raw white mixture on the day of chemotherapy, 40mL once, 3 times a day, for 4 days, and start taking half the dose on the 5th day, 20ml once, 3 times a day, for 4 days, a total of 8 days (a total of 3 bottles of raw white mixture). If there is a decrease in leukocytes/neutrophils at grade IIIc or higher, G-CSF (200 mcg) is used for at least 3 days until the total leukocyte count ≥ 10×109/L.
Control group: Do not take raw white mixture, if there is a decrease in leukocytes/neutrophils at III° or above, use G-CSF (200 μg) for at least 3 days until the total number of leukocytes ≥ 10×109/L.
(1) Changes in peripheral blood leukocytes/neutrophils, check peripheral blood leukocytes every 3 days, leukocytes are lower than 2.0×109/L or neutrophils are lower than 1.0×109/L, and peripheral blood leukocytes are checked once a day; The toxicity assessment refers to CTCAE4.03.
(2) G-CSF dosage: compare the average dosage of G-CSF between the two groups.
Statistical analysis: SPSS19.0, chi-square test and paired T-test, the test level was P=0.05 (bilateral), P<0.05 was statistically significant.
Findings:
Shengbai mixture significantly reduced the incidence of myelosuppression
ACCORDING TO CTCAE4.03, bone marrow toxicity was mainly manifested by a decrease in peripheral blood leukocytes and neutrophils, and the incidence of grade III~IV myelosuppression after chemotherapy in the treatment group (white group) was 8.42%, which was significantly lower than that in the control group (22.9% (Χ2=8.52, P<0.01). Shengbai mixture can reduce the incidence of myelosuppression after chemotherapy by 63.23%, and effectively prevent myelosuppression after chemotherapy.
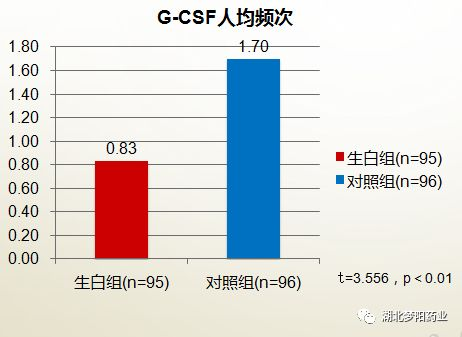
The whitening mixture significantly reduced the amount of G-CSF
In the treatment group (raw white group), 24 patients were treated with G-CSF for myelosuppression, and 79 G-CSF (200 μg) were shared. In the control group, 53 patients were treated with G-CSF for myelosuppression, and 163 G-CSF (200 μg) were shared. The per capita dosage of G-CSF in both groups was 0.83 (79/95) and 1.70 (163/96), respectively (t=3.566, P<0.01). It can be seen that the raw white mixture can significantly reduce the amount of G-CSF used in chemotherapy.
The results of the study showed that the combined application of raw white mixture in the course of lung cancer chemotherapy could:
1. Significantly reduce the incidence of myelosuppression;
2. Reduce the amount of G-CSF used during chemotherapy;
3. Reduce the toxicity and side effects of chemotherapy drugs, improve the quality of life of patients, and increase chemotherapy tolerance;
4. Compared with other studies, Zhao Zefeng et al. [1] summarized the role of drugs such as Diyusheng Baipian in the prevention of myelosuppression, but our study confirmed that the effect of Shengbai Mixture in preventing bone marrow suppression was better than that of Diyusheng Baipiao.
References:
[1] Zhao Zefeng, He Xirui, Zhang Qiang, et al. Meta-analysis of leukopenia caused by chemotherapy in the treatment of tumors[J].Northwest Pharmaceutical Journal,2017,(5):648-652.





COPYRIGHT© 2022 Hubei Mengyang Pharmaceutical Co., LTD. All Rights Reserved E ICP 11014915; Internet Drug Information Service Certificate No. : (E) - Non-business -2016-0032
Technical Support: SUMA
